Huge fires broke out during this year. Most notably, California in the United States, British Columbia and northern Ontario have also suffered the consequences of Mother Nature. The great changes that our planet is undergoing are causing upheaval in wildlife in general, but also in living environments. Forest fires are usually the cause of poor air quality in affected cities. In addition, its odor is very persistent as it succeeds in becoming embedded in houses.
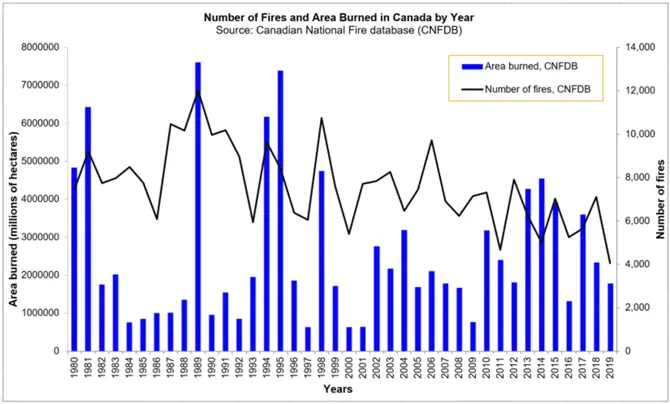
According to data compiled in the National Forest Database, more than 8,000 wildfires occur in Canada each year, destroying an average of over 2.1 million hectares. In addition, lightning causes almost 50% of all fires, but is responsible for some 85% of the area burned annually. (1)
The figure shows statistics extracted from the CNFDB and provides a comparison with those numbers reported annually to the National Forestry Database (NFD). This chart shows the high variability in both number of fires and area burned in Canada per year. Note that the data contained in the CNFDB are not complete nor are they without error. Not all fires have been mapped, and data accuracy varies due to different mapping techniques. This collection includes only data that has been contributed by the agencies. Data completeness and quality vary among agencies and between years.
Wildfires often start unnoticed, but can spread at lightning speed and consume huge areas, igniting bushes, trees, houses and buildings in their path. Debris from such a fire can be thrown up to two kilometers away, while sparks and embers can ignite nearby homes and materials and cause extensive damage. (1) Large blazes devour entire regions, causing gigantic plumes of smoke to sweep across Canada from west to east.
Smoke is damaging to individuals and their health in general. Several factors explain these impacts such as the health of people, the amount of smoke embedded in the house and the concentration of smoke from nearby forest fires, as smoke is harmful. Clearly, the length of time you are exposed to this harmful smoke plays a critical role in the future impacts on your health. (2)
What are the components of smoke?
First, they are fine particles that scatter light and make smoke visible. These make it difficult to breathe and can cause a strong cough. These fine particles can penetrate deep into the lungs and worsen pre-existing heart and respiratory disease. (2)
In addition, smoke also contains a wide variety of volatile organic compounds (VOCs) which are invisible gas molecules. Several of these molecules are known to be carcinogenic. These are PAHs (Polycyclic Aromatic Hydrocarbons), such as pyrenes, benzenes, and dioxins. They also often come in the form of aerosols, i.e. liquids suspended in the air, commonly known as creosotes. These are substances that are particularly harmful to health.
Who is most likely to be affected by these fumes?
Young children, the elderly, and people with heart or lung conditions such as asthma, chronic bronchitis, emphysema and congestive heart failure are more susceptible to the harmful effects of exposure to smoke. People who participate in sports or do strenuous work outdoors may also be more vulnerable because they breathe more deeply and quickly. The denser the smoke and the longer the exposure, the greater the risk to those affected. (2)
What are the symptoms of exposure to smoke?
Exposure to smoke can cause eye irritation, tears, coughing, and a runny nose (runny nose). If the smoke lasts for several days to several weeks or if it is really thick, it can result in lung problems and persistent cough (2)
Various solutions are proposed to you to remedy these smoke problems by Sanuvox. Indeed, installing air purification systems can treat the air in these rooms and eliminate odors and smoke particulates.Unlike its competitors, Sanuvox does not use expensive activated carbon filters which have the disadvantage of saturating very quickly and becoming useless. The patented UV process reduces odors and airborne smoke. A recirculation rate of 3 to 6 times per hour makes it possible to choose the appropriate equipment given the dimensions of the room.
The air purification units are equipped with a blower, pre-filter, HEPA filter and UV sources of appropriate wavelengths.
This is to eliminate odors by oxidizing the odor molecules. This is the process that takes place naturally outside in the atmosphere with the rays of the sun. For example, the oxidation of hydrogen sulfide H2S produces a completely odorless H2O water molecule as well as a SO2 sulfur oxide molecule with an odor threshold of 5 mg / Nm3, which is 5,000 times less odorous than the initial hydrogen sulphide molecule.
Called “combustion” when it comes to conventional fuels, such a reaction then requires a high temperature to start. There is another way to initiate chemical oxidation reactions by using light with a high energy intensity photon source. This process of oxidation at room temperature is called “photolysis” or “photo-oxidation”.
The energy of photons, the particles that make up light, increases as the wavelength of light decreases. As a result, having a wavelength of 400 nm, purple photons are more energetic than red photons with a wavelength of 700 nm. The more energy photons have, the more they are like large caliber projectiles that can break the bonds that bind atoms together within a single molecule.
To initiate oxidation reactions, these bonds must first be broken to free the atoms which can then combine with oxygen atoms.
By using ultraviolet light sources with wavelengths of 254 nm for UV-C and 185 nm for UV-V, respectively, it is possible to emit photons with energies large enough to break and then to oxidize almost any scent molecule. Here is an example of the steps of the photo-oxidation process of hydrogen sulfide.
The first step is to break the chemical bond between the sulfur atom S and the hydrogen atom. The energy of this bond well known to chemists is 347 kJ / mole as shown in the attached Table 2, while the energy of UV-C photons is 470 kJ / mole. UV-C photons will therefore have no difficulty in breaking these bonds and momentarily releasing the sulfur and hydrogen atoms.
The second step is to provide oxygen atoms to react with the atoms thus released. Ambient air contains a lot of oxygen atoms (almost 21% of the air, the rest being nitrogen), but they ring in molecular pairs whose bond energy holding them together two by two is of 495 kJ / mole. In this case, UV-C photons (470 kJ / mol) lack the energy to break the bond and release oxygen atoms. It will be necessary to use a more energetic source of photons. This is the case with UV-V photons at wavelength 185 nm, whose energy of 646 kJ / mole easily exceeds the binding energy of an oxygen molecule (495 kJ / mole).
Once these two steps are completed, the oxidation reaction will occur at room temperature via a hydroxyl radical (OH *) which acts as a transmission intermediary for the free oxygen atoms.
The end result is the conversion and neutralization by oxidative effect of strongly odorous molecules into other molecules with little or no odor. The end products of this photo-oxidation process which in fact accelerates the natural process are then oxidized molecules and non-sticky dry ash particles which can now be captured by filters without causing them to clog. In this way, odors are removed by the oxidation process and the resulting dry particles are removed by filtration.
Besides conventional filtration, there is another well-known way to remove solid particles from the air. Electrostatic filters, also often called air ionizers, have somehow this ability. Instead of mechanically capturing particles like conventional filters, the principle of operation of ionizers is to electrically charge the particles so that they migrate under the effect of electrical forces to neighboring surfaces. The same effect is achieved by rubbing a balloon on hair, then sticking it to a wall. After a while, however, the particle loses its charge and becomes airborne again. This is therefore a fairly temporary solution which in fact only stirs up the dust inside the house. This is why we dismissed ionizers as a method to really remove smoke particulates.
This article has described in detail the impact on the health, nature and composition of smoke in general and the inherent disadvantages of conventional filtration and air ionizers. Many years of experimental studies based on the chemical properties of smoke have shown that the smell of smoke cannot be removed without changing the structure of the molecules responsible for the odor. Besides thermal incineration, ultraviolet photooxidation has been shown to be the most effective way to achieve this by degrading these molecules through oxidation. Their oxidation makes smoke particles and aerosols dry and non-sticky, making them eligible candidates for standard filtration.
Thanks to the following references and sources:
(1) National Forest Database (BDNF)
(2) Government of Manitoba
(3) Hays, Gobbell, Ganick, Indoor Air Quality, McGraw-Hill, 1995, p.58.
(4) Spengler, Samet, McCarthhy, Indoor Air Quality Handbook. McGraw-Hill, 2001.
(5) UWaterloo, Bond Lengths and Energies. n.d. Web. 21 Nov 2010.
(6) http://www.science.uwaterloo.ca/~cch…20/bondel.html EPA. Reference Guide to Odor Thresholds for Hazardous Air Pollutants Listed in the Clean Air Act Ammendments of 1990.
EPA / 600 / R-92/047, March 1992